REGULATORY
COMPLIANCE
COMPLIANCE
WITHOUT
THE CHAOS.
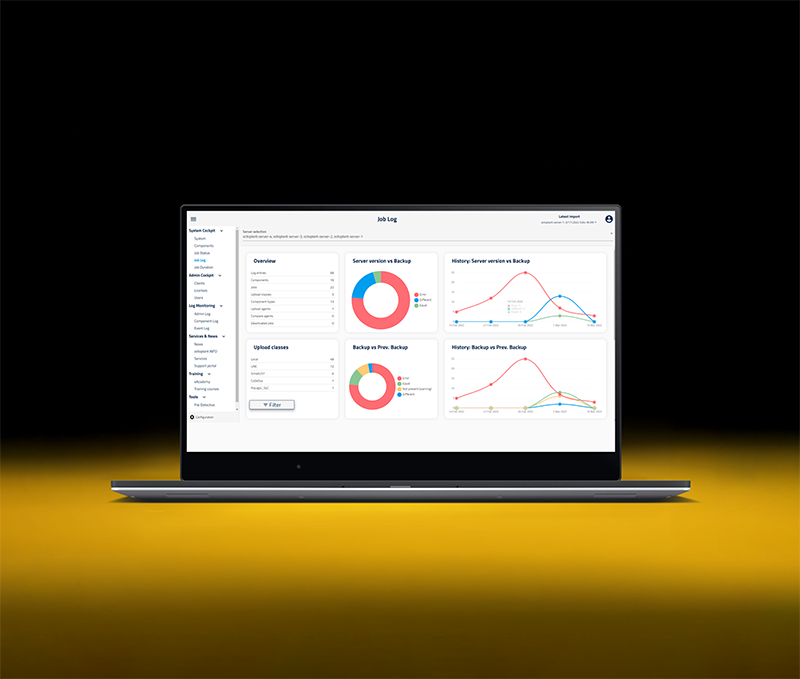
Octoplant simplifies regulatory compliance by automating the documentation, traceability, and workflows required to meet industry-specific standards like FDA, GxP, ISO, and more. In highly regulated manufacturing sectors, proving what was changed, by whom, and under what conditions isn’t optional – it’s essential. Octoplant builds audit-ready records as part of your everyday OT maintenance, eliminating the need for manual logbooks or post-event reconstructions. With clear version histories, user access tracking, and enforced process controls, it helps organizations consistently meet internal policies and external regulations with less effort and greater confidence.
Octoplant supports alignment with NIS2 and NIS-T requirements by delivering transparent change histories, role-based access controls, and standardized incident documentation. These features help utilities, logistics providers, and other critical infrastructure operators demonstrate cybersecurity resilience, ensure operational continuity, and provide the evidence required for timely incident reporting under these directives.
change history
generation
workflows
access
and
notifications
Integration
audit reports